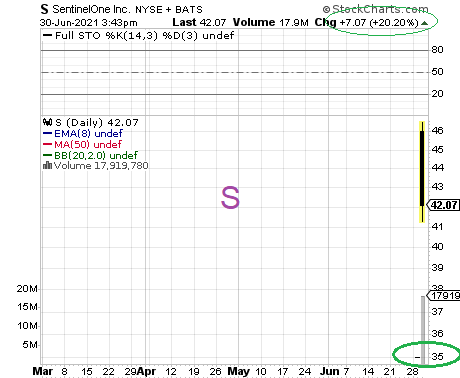
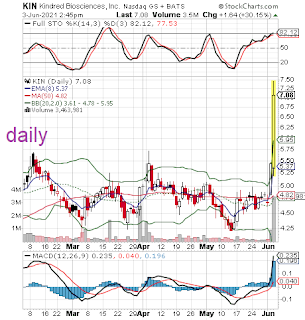
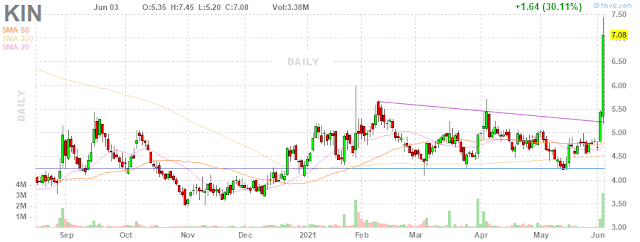
SAN
FRANCISCO, June 2, 2021 /PRNewswire/ -- Kindred Biosciences, Inc.
(NASDAQ: KIN), a biopharmaceutical company focused on saving and
improving the lives of pets, today announced positive results from a
pivotal efficacy study of KIND-030 in dogs infected by parvovirus. The
primary endpoint was survival and the results showed 100% survival in
the treated group versus 43% survival in the placebo group.
KIND-030, a monoclonal antibody targeting canine parvovirus (CPV), is partnered with Elanco Animal Health (NYSE: ELAN).
In
this randomized, blinded, placebo-controlled study, KIND-030 was
administered to dogs after they tested positive for CPV infection. The
primary endpoint of the study was met. The parvovirus challenge resulted
in 57% mortality rate in the control dogs compared to 0% mortality rate
in the KIND-030 treated dogs. The dogs did not receive any supportive
care or other treatments.
"Parvovirus is a
devastating disease currently without any available treatment," said
KindredBio's Chief Executive Officer, Richard Chin, M.D. "With 100%
efficacy, we believe KIND-030 has the potential to revolutionize the
care of these dogs. Instead of a lengthy and expensive hospitalization
that is frequently ineffective and can leave the dog with permanent
disabilities, the infected dogs can now be treated with a single
injection without need for additional supportive care or
hospitalization."
"We are excited to partner
with KindredBio on this revolutionary treatment that can significantly
improve the health and well-being of dogs," said Jeff Simmons, president
and CEO of Elanco Animal Health. "With our significant global reach and
access to veterinarians and pet owners around the world, Elanco looks
forward to leveraging our capabilities and skilled team of experts to
advance and commercialize this novel treatment for pets globally."
With
this positive study, KIND-030 has now demonstrated efficacy in both
indications being pursued: prophylactic therapy to prevent clinical
signs of canine parvovirus infection, and treatment of established
parvovirus infection. Results from the pivotal efficacy study for the
therapeutic claim are expected to be submitted to the United States
Department of Agriculture (USDA) in June, with possible approval by
year-end 2021.
CPV is the most significant and
contagious viral cause of enteritis in dogs, especially puppies, with
mortality rates as high as 91% if untreated. There are currently no Food
and Drug Administration or USDA approved treatments for CPV, nor any
other available treatment. Veterinary intervention is limited to
supportive care, which can cost owners up to thousands of dollars per
puppy, with an average cost of $1,200.
Canine
parvovirus is most often seen in puppies less than 6 months of age, but
can occur in unvaccinated dogs of any age. Clinical signs often include
depression, not eating, vomiting, and profuse diarrhea which is often
blood tinged.1 Banfield estimates that there are approximately 250,000
parvo cases in the U.S. each year, excluding emergency hospitals,
shelters, specialty hospitals, or undiagnosed cases.2
KIND-030 binds to critical portions of the virus, preventing the virus from entering into cells.
On
April 28, 2021 KindredBio announced acceptance of the parvovirus
antibody prophylaxis study data and approval of the efficacy indication
by the USDA Center for Veterinary Biologics. The pivotal efficacy data
for the prophylactic indication demonstrated that 0% of the KIND-030
treated dogs developed parvovirus infection while 100% of the
placebo-control dogs developed the disease, and also showed 100%
survival rate in KIND-030.
Regulatory approval
and review timeline are subject to the typical risks inherent in such a
process. The results of the pivotal efficacy study for the therapeutic
claim stated in this press release have not been reviewed by the USDA
Center for Veterinary Biologics.