- Tirzepatide is an active ingredient of Eli Lilly’s popular Zepbound and Mounjaro.
- The trial was designed to evaluate the efficacy as measured by body weight reduction and other outcomes, safety, and tolerability of two oral doses of azelaprag (300 mg, once or twice daily) in combination with tirzepatide (5 mg subcutaneous injection once weekly).
- In parallel to evaluating azelaprag, Company will continue to advance earlier platform-derived programs, including IND submission for CNS penetrant NLRP3 inhibitor anticipated in the second half of 2025.
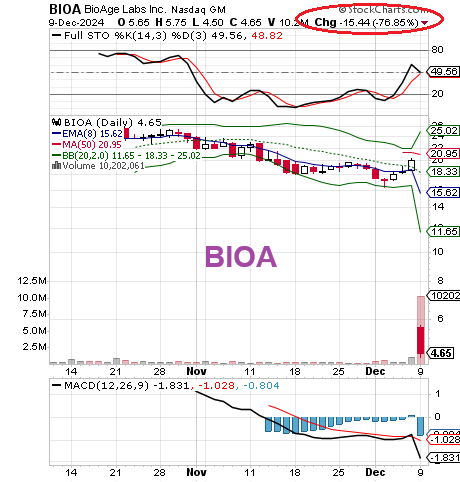
On Friday, BioAge Labs Inc. (NASDAQ:BIOA) announced that it will discontinue the ongoing STRIDES Phase 2 study of its investigational drug candidate, azelaprag.
STRIDES was being conducted in collaboration with Eli Lilly And Co’s (NYSE:LLY) Chorus clinical development organization. Top-line results were anticipated in the third quarter of 2025.
The move follows after liver transaminitis without clinically significant symptoms was observed in some subjects receiving azelaprag.
STRIDES is a Phase 2 clinical trial of azelaprag as monotherapy and in combination with tirzepatide that planned to enroll approximately 220 individuals with obesity aged 55 years and older.
Tirzepatide is an active ingredient of Eli Lilly’s popular Zepbound and Mounjaro.
The trial was designed to evaluate the efficacy as measured by body weight reduction and other outcomes, safety, and tolerability of two oral doses of azelaprag (300 mg, once or twice daily) in combination with tirzepatide (5 mg subcutaneous injection once weekly).
An azelaprag monotherapy arm was included to provide additional safety information.
The company said no transaminase elevations were observed in the tirzepatide only treatment group.
Of 204 subjects enrolled in STRIDES, 11 subjects in the azelaprag treatment groups were observed to have transaminase (liver enzymes) elevations with no clinically significant symptoms. Dosing of all subjects will be discontinued, and no additional subjects will be enrolled.
Clinical follow-up of enrolled subjects will continue off-drug. The company intends to further analyze available STRIDES clinical data from all enrolled subjects and share updated plans for azelaprag in the first quarter of 2025.
The company’s brain-penetrant NLRP3 inhibitors are progressing toward IND submission, anticipated in the second half of 2025. The NLRP3 inhibitor program targets neuroinflammation linked to metabolic and neurodegenerative diseases.
As of September 30, BioAge had approximately $334.5 million in cash and cash equivalents, which is expected to be sufficient to fund operations and capital expenses into 2029.
In October, BioAge Labs closed its upsized initial public offering of 12.65 million shares at $18 per share.