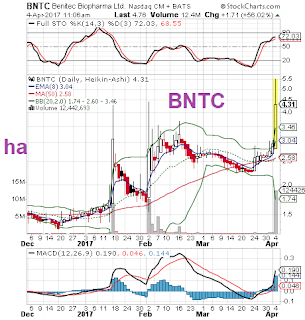
Ticker: BNTC
Benitec Biopharma announces that the initial pre-clinical efficacy results of the OPMD program have been published in Nature Communications; studies demonstrate that a DNA directed RNA interference (ddRNAi) approach to 'silence and replace' the mutant PABPN1 protein:
- Co announces that the initial pre-clinical efficacy results of the OPMD program have been published in Nature Communications, an open access scientific journal published by the Nature Publishing Group. OPMD, a rare progressive muscle-wasting disease caused by mutation in the poly-binding protein nuclear 1 gene, is characterised by eyelid drooping, swallowing difficulties, and proximal limb weakness.
- The key results from these studies demonstrate that a DNA directed RNA interference approach to 'silence and replace' the mutant PABPN protein, results in the correction of the muscular dystrophy and of key clinical features of OPMD including a progressive atrophy and muscle weakness associated with nuclear aggregates of insoluble PABPN1. These data were generated in the A17 mouse model that expresses the mutant PABPN1 gene and mimics most of the features of human OPMD patients.
Description
Benitec Biopharma Limited is a biotechnology company. The Company is engaged in progressing programs through the clinic; the commercialization of its Intellectual Property (IP); development of its therapeutic pipeline and pre-clinical programs, and funding, and protecting and building the IP estate. Its In-house product candidates include TT-034, BB-HB-331, BB-AMD-211 and ddRNAi therapeutic. It is focused on commercialization by licensing and partnering of patents and licenses in biotechnology, in functional genomics, with applications in biomedical research and human therapeutics. It has a pipeline of in-house and partnered therapeutic programs based on its patented gene-silencing technology, deoxyribonucleic acid (DNA)-directed ribonucleic acid interference (ddRNAi). The Company is engaged in developing treatments for chronic human conditions, such as hepatitis B, wet age-related macular degeneration (AMD), and oculopharyngeal muscular dystrophy (OPMD) based on this technology.





No comments:
Post a Comment